Health
EU Defends COVID Vaccine Approvals Against Misleading Claims
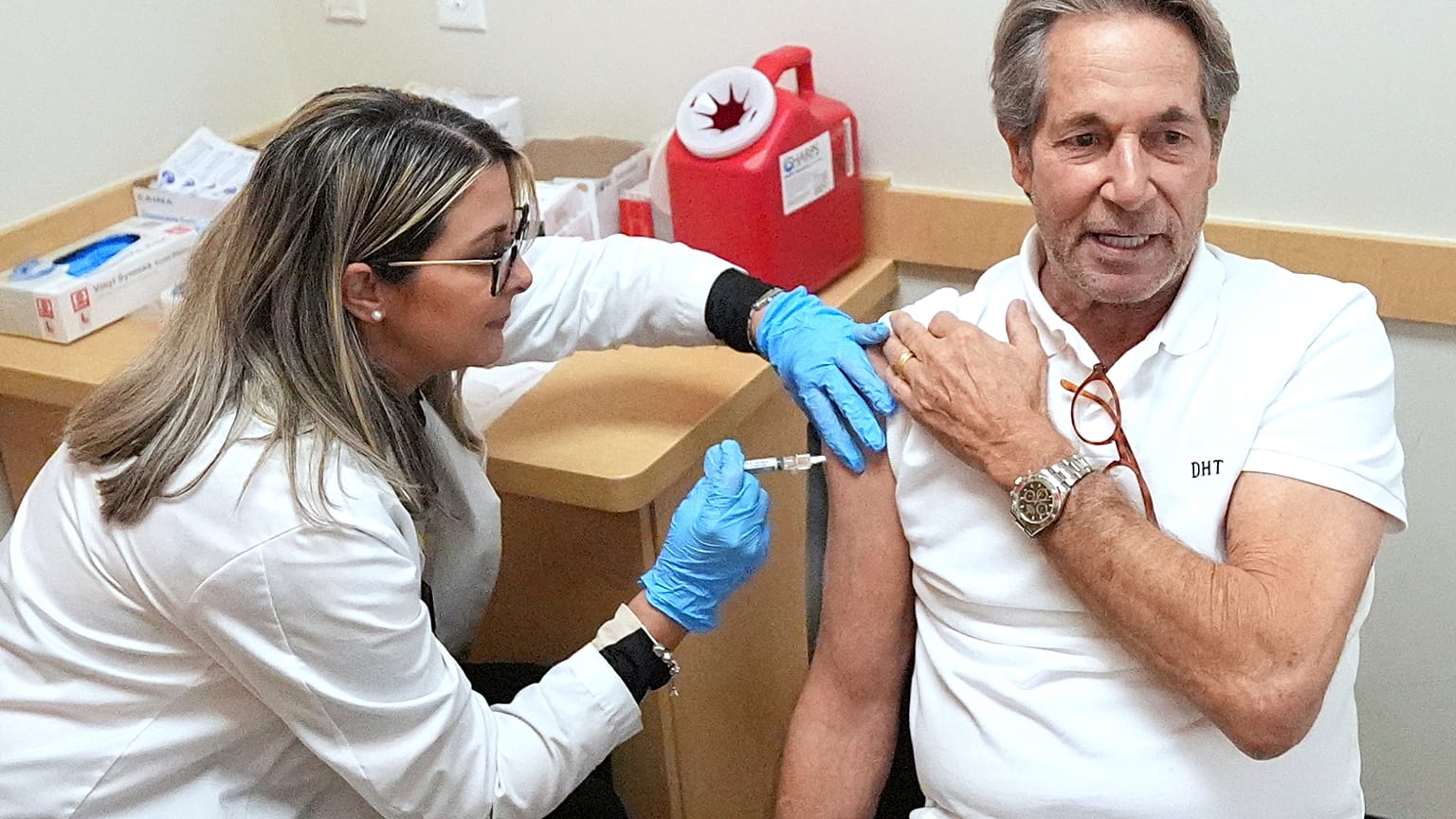
Misleading claims regarding the approval of COVID-19 vaccines are circulating on social media, provoking confusion among the public. A recent statement from the European Commission has been misinterpreted, suggesting that vaccines received clearance without adequate safety checks. These assertions have caught the attention of various media outlets and influencers, leading to a wave of misinformation.
The controversy began when a video by YouTuber CharOfficiel gained traction, with over 16,000 views. He referenced an article from the German newspaper Berliner Zeitung, published on September 16, 2025. The article claimed that the European Commission admitted that COVID-19 vaccines were authorized without comprehensive safety data. Citing a question from Gerald Hauser, an Austrian Member of the European Parliament (MEP) from the far-right Patriots for Europe group, the article suggested a significant lapse in the vaccine approval process.
Hauser’s inquiry focused specifically on the Comirnaty vaccine, developed by BioNTech and Pfizer, which was the first COVID-19 vaccine authorized in Europe. He questioned why the Commission did not inform citizens that the efficacy and safety of the gene-based vaccine were not guaranteed, as stipulated in the contract.
Understanding Conditional Marketing Authorisation
In response to Hauser, the European Commission clarified that COVID-19 vaccines were granted a “conditional marketing authorisation.” This regulatory framework allows for the approval of medicines that address unmet medical needs, even when comprehensive data are not yet available. It is important to note that such approvals are not unique to COVID-19 vaccines; they have been used in other public health emergencies, such as the Ebola outbreak.
A spokesperson for the Commission explained that conditional marketing authorisations permit the release of medicines based on less comprehensive clinical data than typically required. This approach is justified when the benefits of immediate access to the vaccine outweigh the risks associated with incomplete data. The spokesperson emphasized that these authorisations were not blanket approvals; thousands of individuals participated in clinical trials as part of the assessment process conducted by the European Medicines Agency (EMA).
The Commission’s response has been misinterpreted in some instances, leading to claims that it represents a new admission of oversight. However, the fact that COVID-19 vaccines were fast-tracked during a global health crisis has been widely known. Once the vaccines received full marketing authorisations, the EMA and EU member states continued to monitor reports of suspected side effects, maintaining the ability to revoke authorisations if necessary.
Impact of COVID-19 Vaccines
Despite the controversy surrounding their approval, studies indicate that COVID-19 vaccines have had a significant impact on public health. According to research published in the Lancet Respiratory Medicine, the vaccines reduced deaths by approximately 59% between December 2020 and March 2023, translating to roughly 1.6 million lives saved.
The ongoing discourse surrounding vaccine approvals highlights the critical importance of clear communication from health authorities. As misinformation spreads, it is essential for regulatory bodies to reinforce their commitment to transparency and public safety in the approval process for vaccines and other medicines.
-

 Top Stories3 months ago
Top Stories3 months agoTributes Surge for 9-Year-Old Leon Briody After Cancer Battle
-

 Entertainment4 months ago
Entertainment4 months agoAimee Osbourne Joins Family for Emotional Tribute to Ozzy
-

 Politics4 months ago
Politics4 months agoDanny Healy-Rae Considers Complaint After Altercation with Garda
-
 Top Stories4 months ago
Top Stories4 months agoIreland Enjoys Summer Heat as Hurricane Erin Approaches Atlantic
-

 World5 months ago
World5 months agoHawaii Commemorates 80 Years Since Hiroshima Bombing with Ceremony
-

 Top Stories3 months ago
Top Stories3 months agoNewcastle West Woman Patricia Foley Found Safe After Urgent Search
-

 Top Stories5 months ago
Top Stories5 months agoFianna Fáil TDs Urgently Consider Maire Geoghegan-Quinn for Presidency
-

 World5 months ago
World5 months agoCouple Convicted of Murdering Two-Year-Old Grandson in Wales
-

 World5 months ago
World5 months agoGaza Aid Distribution Tragedy: 20 Killed Amid Ongoing Violence
-

 World5 months ago
World5 months agoAristocrat Constance Marten and Partner Convicted of Infant Murder
-

 Top Stories4 months ago
Top Stories4 months agoClimbing Errigal: A Must-Do Summer Adventure in Donegal
-

 Top Stories4 months ago
Top Stories4 months agoHike Donegal’s Errigal Mountain NOW for Unforgettable Summer Views









