Health
Ireland Poised to Offer State-Funded Weight-Loss Injections
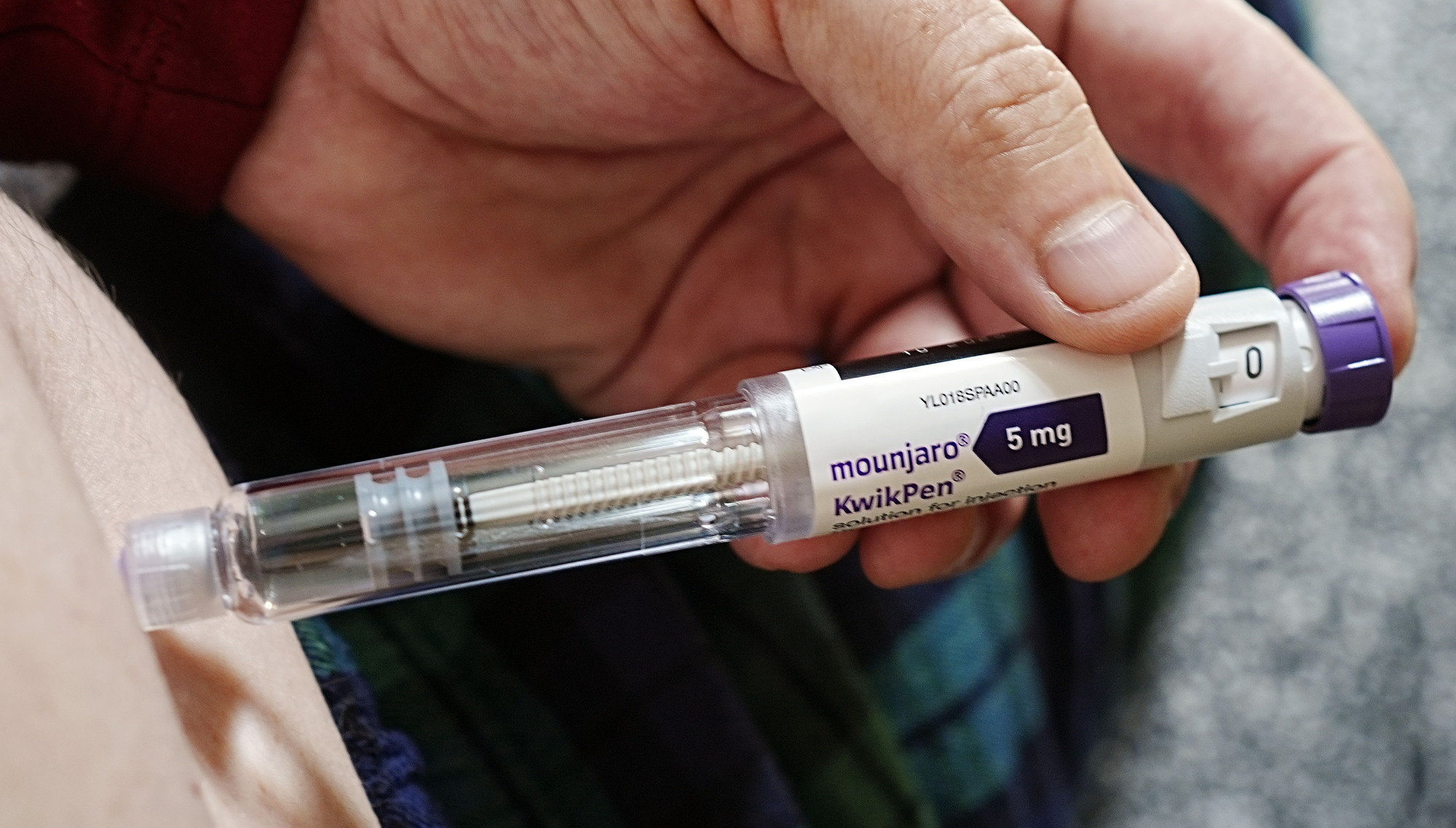
Around 80,000 individuals in Ireland classified as obese may soon qualify for State-funded weight-loss injections if certain medications gain approval. The Health Service Executive (HSE) is currently assessing the potential inclusion of these treatments, including the drugs Mounjaro and Wegovy, for medical card holders or those under the HSE’s Drug Payment Scheme.
The HSE’s national clinical lead for obesity, Professor Donal O’Shea, announced that a health technology assessment (HTA) for Mounjaro will be published in the coming weeks. This assessment will evaluate the medical, social, ethical, and economic implications of the drug, which is a crucial step before determining eligibility for treatment. “This is the precursor to making a decision about who will get it,” Prof. O’Shea stated.
Currently, medications like Mounjaro are available under public schemes for individuals with diabetes. However, several manufacturers, including Novo Nordisk and Eli Lilly, have received approval from the European Medicines Agency (EMA) to market their products for weight loss. These companies are pursuing HSE reimbursement, which would potentially render the treatments free for medical card holders or limit costs to €80 per month under the Drug Payment Scheme.
In March 2025, Novo Nordisk applied for HSE reimbursement for its Wegovy injection, aimed at both adults and adolescents. Presently, Wegovy is not covered under the public scheme for weight-loss patients and is priced at approximately €240 per month for initial dosages, escalating to €320 and €355 for higher dosages.
Eli Lilly has also submitted a reimbursement application for Mounjaro, applicable for weight-loss treatment alongside diabetes management. Some Irish pharmacies currently list Mounjaro starting at €275 per month, with the highest doses costing as much as €595 monthly.
Health Minister Jennifer Carroll MacNeill indicated that the application for Mounjaro could gain approval by the end of the year. Prof. O’Shea explained that the HTA will likely restrict initial access to those in high-risk categories due to the significant financial implications for the HSE. He expressed concern over the barriers faced by individuals seeking effective and safe treatments.
“The bottom line is you have 25% of the population living with obesity, translating to 1.2 million people,” Prof. O’Shea remarked. “They simply can’t begin by letting it be open to everybody, just on a cost basis. We have to make sure we start using it in, initially, the high-risk group and then widen out its use.”
Prof. O’Shea emphasized his desire for broader access to these treatments while advocating for a sustainable introduction that will not impose an overwhelming financial burden. As the situation develops, the outcomes of the HTA will play a pivotal role in shaping the future of weight-loss treatment accessibility in Ireland.
-

 Top Stories2 months ago
Top Stories2 months agoTributes Surge for 9-Year-Old Leon Briody After Cancer Battle
-

 Entertainment4 months ago
Entertainment4 months agoAimee Osbourne Joins Family for Emotional Tribute to Ozzy
-

 Politics4 months ago
Politics4 months agoDanny Healy-Rae Considers Complaint After Altercation with Garda
-
 Top Stories3 months ago
Top Stories3 months agoIreland Enjoys Summer Heat as Hurricane Erin Approaches Atlantic
-

 World4 months ago
World4 months agoHawaii Commemorates 80 Years Since Hiroshima Bombing with Ceremony
-

 Top Stories2 months ago
Top Stories2 months agoNewcastle West Woman Patricia Foley Found Safe After Urgent Search
-

 Top Stories4 months ago
Top Stories4 months agoFianna Fáil TDs Urgently Consider Maire Geoghegan-Quinn for Presidency
-

 World4 months ago
World4 months agoCouple Convicted of Murdering Two-Year-Old Grandson in Wales
-

 World4 months ago
World4 months agoGaza Aid Distribution Tragedy: 20 Killed Amid Ongoing Violence
-

 World4 months ago
World4 months agoAristocrat Constance Marten and Partner Convicted of Infant Murder
-

 Top Stories3 months ago
Top Stories3 months agoClimbing Errigal: A Must-Do Summer Adventure in Donegal
-

 Top Stories3 months ago
Top Stories3 months agoHike Donegal’s Errigal Mountain NOW for Unforgettable Summer Views









