Health
EU Approves Twice-Yearly HIV Injection as Major Medical Advance
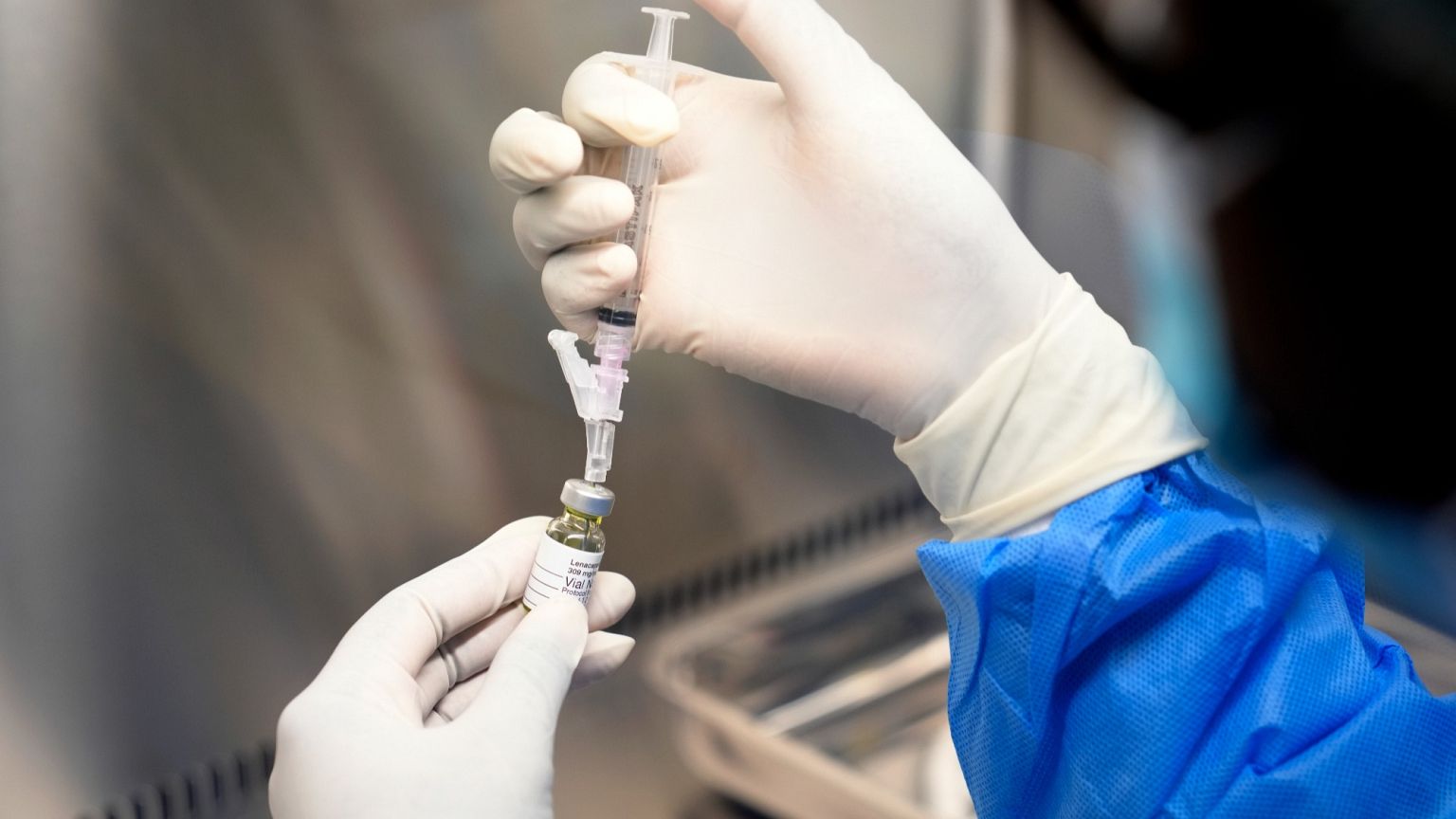
Regulators in the European Union have granted approval for a groundbreaking twice-yearly injection aimed at preventing HIV, which is set to transform the landscape of HIV prevention. The injectable drug, named lenacapavir, developed by Gilead Sciences, is expected to receive formal endorsement from the European Commission later this year.
This innovative treatment represents a significant advancement in the ongoing battle against HIV, offering an alternative to daily oral medications. Clinical studies have demonstrated that lenacapavir is 100 percent effective at preventing the virus, positioning it as a vital component of pre-exposure prophylaxis (PrEP). This method works by inhibiting the virus’s ability to replicate and spread within the human body, significantly lowering the risk of HIV acquisition among both adults and adolescents.
The positive opinion issued by the European Medicines Agency (EMA) advisory committee on March 15, 2024, has paved the way for this essential development in public health. “This milestone reflects our commitment to reimagine HIV prevention in Europe and around the world,” stated Dr. Dietmar Berger, Gilead’s chief medical officer. He emphasized that lenacapavir for PrEP could become a crucial tool for expanding prevention options, particularly for individuals facing significant barriers to care.
Despite progress in HIV treatment and prevention, new cases continue to rise. In 2023, there were over 24,700 new HIV diagnoses reported across the European Union, as well as in Iceland, Liechtenstein, and Norway, marking an 11.8 percent increase from the previous year. The urgency for effective prevention methods is underscored by these statistics, spotlighting the need for innovative solutions.
Gilead has also secured approval from the U.S. Food and Drug Administration (FDA) for lenacapavir, which will be marketed in the EU under the name Yeytuo. In a commitment to global health, Gilead plans to provide generic versions of the drug to 120 lower-income countries with high HIV prevalence. However, concerns remain regarding the drug’s accessibility in light of recent reductions in U.S. funding for global health initiatives.
The EMA and the European Commission did not immediately respond to requests for further comments regarding the implications of this approval. The introduction of lenacapavir marks a promising development in the fight against HIV, with the potential to significantly impact public health strategies across Europe and beyond.
-

 Top Stories3 months ago
Top Stories3 months agoTributes Surge for 9-Year-Old Leon Briody After Cancer Battle
-

 Entertainment4 months ago
Entertainment4 months agoAimee Osbourne Joins Family for Emotional Tribute to Ozzy
-

 Politics4 months ago
Politics4 months agoDanny Healy-Rae Considers Complaint After Altercation with Garda
-
 Top Stories4 months ago
Top Stories4 months agoIreland Enjoys Summer Heat as Hurricane Erin Approaches Atlantic
-

 World5 months ago
World5 months agoHawaii Commemorates 80 Years Since Hiroshima Bombing with Ceremony
-

 Top Stories3 months ago
Top Stories3 months agoNewcastle West Woman Patricia Foley Found Safe After Urgent Search
-

 Top Stories5 months ago
Top Stories5 months agoFianna Fáil TDs Urgently Consider Maire Geoghegan-Quinn for Presidency
-

 World5 months ago
World5 months agoCouple Convicted of Murdering Two-Year-Old Grandson in Wales
-

 World5 months ago
World5 months agoGaza Aid Distribution Tragedy: 20 Killed Amid Ongoing Violence
-

 World5 months ago
World5 months agoAristocrat Constance Marten and Partner Convicted of Infant Murder
-

 Top Stories4 months ago
Top Stories4 months agoClimbing Errigal: A Must-Do Summer Adventure in Donegal
-

 Top Stories4 months ago
Top Stories4 months agoHike Donegal’s Errigal Mountain NOW for Unforgettable Summer Views